Which Of The Following Animals Can Achieve A Net Gain Of H2o From Drinking Seawater?
Dissolved oxygen (DO) is the amount of oxygen that is present in the water. It is measured in milligrams per liter (mg/L), the number of milligrams of oxygen dissolved in a liter of water.
Why is dissolved oxygen important?
Just similar humans, all of the Chesapeake Bay'southward living creatures—from the fish and venereal that swim through its waters to the worms that bury themselves in its muddy bottom—need oxygen to survive.
Humans employ their lungs to inhale oxygen from the air. But worms, fish, crabs and other underwater animals apply gills to get oxygen from the water. As water moves across an animal'southward gills, oxygen is removed and passed into the blood.
Gills work better when there is more than oxygen in the surrounding water. As dissolved oxygen levels subtract, it becomes harder for animals to get the oxygen they need to survive.
How much dissolved oxygen do animals need?
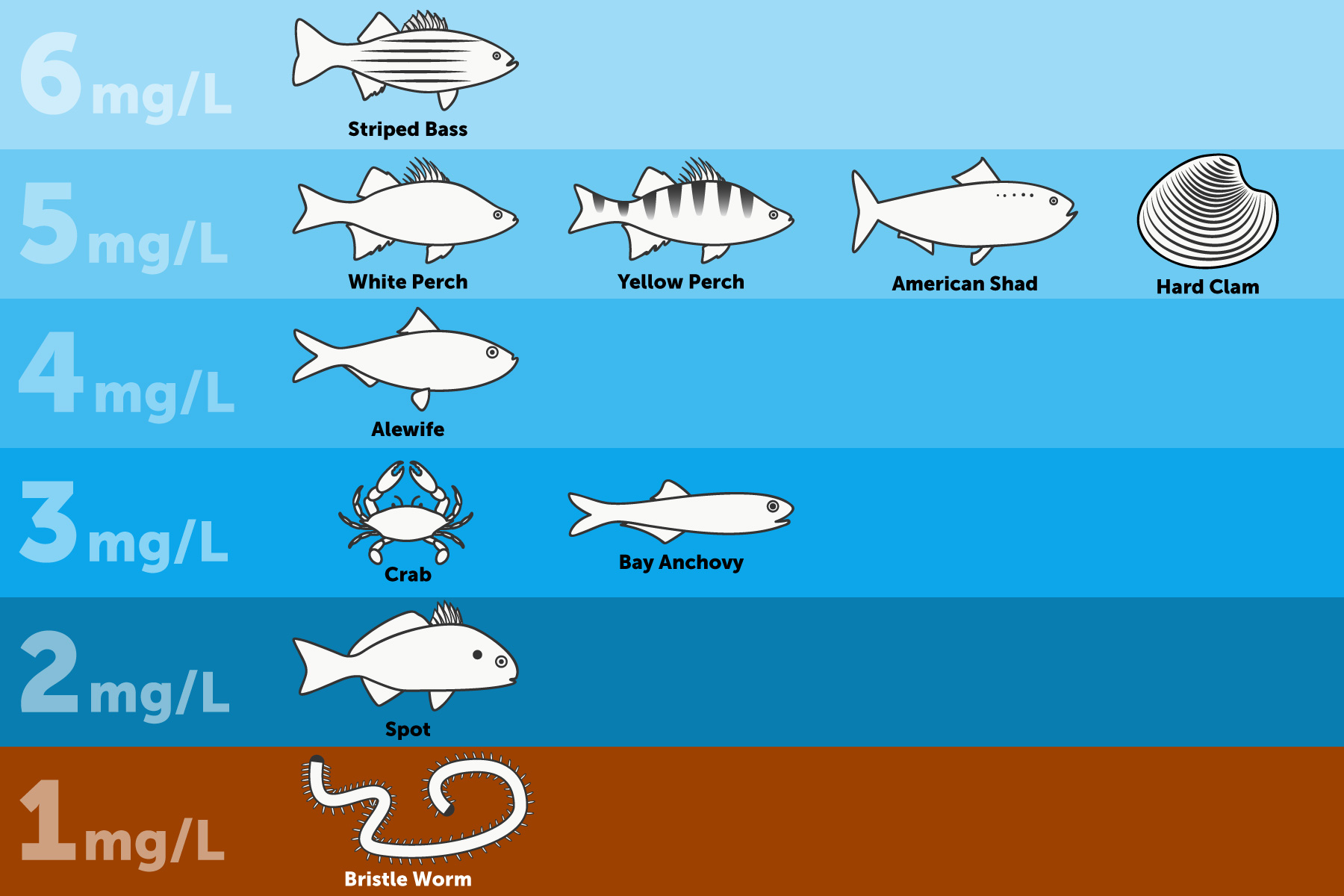
Scientists generally agree that the Bay's critters need dissolved oxygen concentrations of 5.0 mg/Fifty or more to alive and thrive. However, the amount of oxygen an fauna needs varies depending on how large or complex the brute is and where information technology lives.
- Worms and clams that alive in the Bay's muddy lesser—where oxygen levels are naturally low—need dissolved oxygen concentrations of at least 1 mg/L.
- Fish, crabs and oysters that live or feed along the bottom require dissolved oxygen concentrations of 3 mg/L or more.
- Spawning migratory fish and their eggs and larvae need up to half-dozen mg/L during these sensitive life stages.
To see dissolved oxygen levels throughout the Chesapeake Bay, visit Eyes on the Bay (for Maryland waters) or Virginia Estuarine and Coastal Observing System (for Virginia waters).
How does oxygen go into the water?
Oxygen can get into the h2o in several ways:
- Oxygen from the atmosphere dissolves and mixes into the h2o's surface.
- Algae and underwater grasses release oxygen during photosynthesis.
- H2o flows into the Bay from streams, rivers and the ocean. Ocean waters generally take more oxygen. River waters are fast-moving, which helps oxygen from the air mix in.
How exercise low-oxygen areas form?
Hypoxic, or depression-oxygen, areas are regions with less than 2 mg/L of dissolved oxygen. Anoxic, or no-oxygen, areas are regions with less than 0.2 mg/L of dissolved oxygen. These areas are frequently called "dead zones," because well-nigh animals cannot survive there. Areas in the Bay that have low dissolved oxygen levels are the event of a complex interaction of several natural and man-made factors, including temperature, food pollution, h2o flows and the shape of the Bay'due south lesser.
High temperatures
Temperature limits the amount of oxygen that can dissolve in water: water can hold more oxygen during winter than during the hot summer months. However, fifty-fifty at the warmest temperatures seen in the Bay (around 91 degrees Fahrenheit), water is capable of having dissolved oxygen concentrations of 6 to 7 mg/L. And so, although high temperatures can influence dissolved oxygen levels, temperature is not the merely cause of depression-oxygen areas found in the Bay each summer.
Nutrient pollution
Excess nutrients in the water (known as eutrophication) can fuel the growth of algae blooms. Oysters, menhaden and other filter feeders eat a portion of the excess algae, only much of it does not finish up being consumed. The leftover algae dice and sink to the Bay'southward bottom, where they are decomposed by bacteria. During this process, bacteria eat oxygen until there is picayune or none left in these lesser waters.
Flow of water
The division betwixt water flowing from the bounding main and from the Bay'southward freshwater rivers and streams can also influence dissolved oxygen levels. Water flowing from the ocean is generally salty and cooler, while river water is fresh and warmer. Because of these differences, river h2o weighs less than ocean water and floats on top of it—although current of air and other potent mixing forces may change this pattern.
The boundary where the fresh h2o layer meets the table salt water layer below is called the pycnocline. The pycnocline acts as a concrete barrier that prevents the 2 layers from mixing together. During the summertime, when algae-consuming bacteria are most active, the pycnocline cuts off oxygen-deprived bottom waters from oxygen-rich surface waters. This can create large areas of low- or no-oxygen at the lesser of the Bay.
Shape of the Bay's bottom
The bottom of the Bay is non flat—rather, it has varying shallow and deep areas. In certain basin-shaped areas at the bottom of the Bay, the pycnocline can act like a "hat" that cuts off bottom waters from receiving whatsoever oxygen. This phenomenon frequently takes place each summer in:
- The middle of the Bay'south mainstem, from the Bay Bridge south to the mouth of the Potomac River,
- The lower Chester, Potomac and Rappahannock Rivers, and
- The lower part of Eastern Bay, near Kent Island.
Source: https://www.chesapeakebay.net/discover/ecosystem/dissolved_oxygen
Posted by: blairroyes1951.blogspot.com

0 Response to "Which Of The Following Animals Can Achieve A Net Gain Of H2o From Drinking Seawater?"
Post a Comment